





Copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.
Copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.
All slides copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.
How, why, when, and for what we vaccinate today starts with the story of smallpox, polio, and diphtheria. Our whole understanding of how vaccine immunity works began with the discovery of the relationship between smallpox and cowpox. This one thing alone resulted in an inestimable number of lives saved in the last two hundred years.This course is designed to give accurate information for parents. We recognize (and support) the right to refuse vaccines contrary to medical advice. But we also know that many times, parents seem to opt out because they don't know the facts about what vaccines do, what they actually contain, and why we think they are so important.It is best viewed on a desktop computer, and I strongly encourage you to take notes. I do not suggest that children view the content!After you view all the slides, you'll end up back here where you can click on the button to take the open book quiz. There are 20 questions pulled randomly from a pool of almost 100. Some questions are worth more points. You can view the slides anytime and take the quiz as many times as you need to.
Any video material will NOT be on the quiz. Click the right slide arrow to begin. The last slide has the link to the quiz.
How, why, when, and for what we vaccinate today starts with the story of smallpox, polio, and diphtheria. Our whole understanding of how vaccine immunity works began with the discovery of the relationship between smallpox and cowpox. This one thing alone resulted in an inestimable number of lives saved in the last two hundred years.This course is designed to give accurate information for parents. We recognize (and support) the right to refuse vaccines contrary to medical advice. But we also know that many times, parents seem to opt out because they don't know the facts about what vaccines do, what they actually contain, and why we think they are so important.It is best viewed on a desktop computer, and I strongly encourage you to take notes. I do not suggest that children view the content!After you view all the slides, you'll end up back here where you can click on the button to take the open book quiz. There are 20 questions pulled randomly from a pool of almost 100. Some questions are worth more points. You can view the slides anytime and take the quiz as many times as you need to.
Any video material will NOT be on the quiz. Click the right slide arrow to begin. The last slide has the link to the quiz.
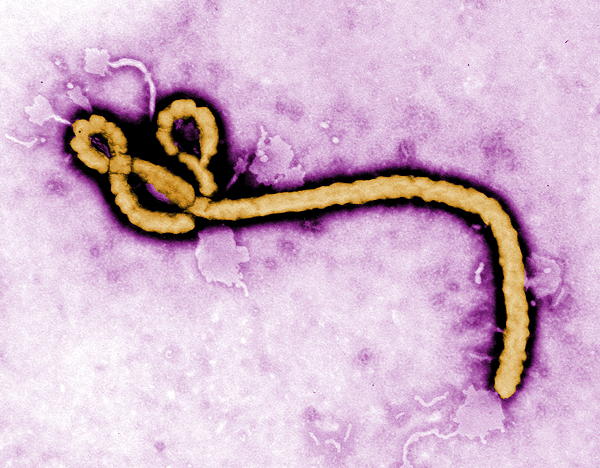
History. Ebola was formerly know as Ebola hemorrhagic fever and has been found in remote African villages, where it is though to have originated in fruit bats, porcupines, and non-human primates, though a proven host of origin is unknown.
Ebola is among a group of filoviruses that have this characteristic, but nontypical filarial form. There are several strains: bundibugyo ebolavirus, bombali ebolavirus, reston ebolavirus, sudan ebolavirus, and Tai Forest ebolavirus, and Zaire ebolavirus.
According to the WHO, the fatality rate averages around 50%, past outbreaks have ranged from 25% to 90%. Typically, though I have heard 70% as the most often quoted number. Recent outbreaks include one in West Africa in 2014-2016, and four different locales of the Democratic Republic of Congo in 2017, 2018, 2018-2020, and 2020.

Dr. Schreiber of San Augustine giving a typhoid inoculation at a rural school, San Augustine County, Texas, in April 1943. This image is a work of an employee of the United States Farm Security Administration or Office of War Information domestic photographic units, taken as part of that person's official duties. As a work of the U.S. federal government, the image is in the public domain in the United States. Background image: This is a transmission electron microscopic (TEM) image of a cluster of smallpox viruses, which had been processed using a negative stain technique. See PHIL 2294 for a black and white version of this digitally-colorized image.
Dr. Schreiber of San Augustine giving a typhoid inoculation at a rural school, San Augustine County, Texas, in April 1943. This image is a work of an employee of the United States Farm Security Administration or Office of War Information domestic photographic units, taken as part of that person's official duties. As a work of the U.S. federal government, the image is in the public domain in the United States. Background image: This is a transmission electron microscopic (TEM) image of a cluster of smallpox viruses, which had been processed using a negative stain technique. See PHIL 2294 for a black and white version of this digitally-colorized image.
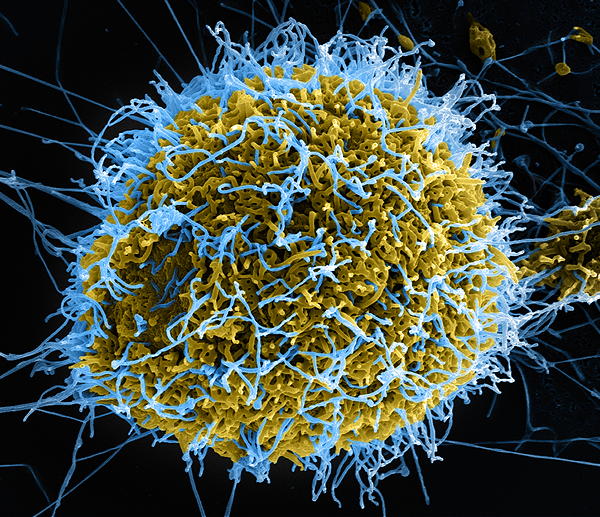
Produced by the National Institute of Allergy and Infectious Diseases (NIAID), this digitally-colorized scanning electron microscopic (SEM) image depicts numerous filamentous Ebola virus particles (blue) budding from a chronically-infected VERO E6 cell (yellow-green). See the Flickr link below, for additional SEM NIAID Ebola virus imagery. Public domain.

Copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.
Copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.
All slides copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.
How, why, when, and for what we vaccinate today starts with the story of smallpox, polio, and diphtheria. Our whole understanding of how vaccine immunity works began with the discovery of the relationship between smallpox and cowpox. This one thing alone resulted in an inestimable number of lives saved in the last two hundred years.This course is designed to give accurate information for parents. We recognize (and support) the right to refuse vaccines contrary to medical advice. But we also know that many times, parents seem to opt out because they don't know the facts about what vaccines do, what they actually contain, and why we think they are so important.It is best viewed on a desktop computer, and I strongly encourage you to take notes. I do not suggest that children view the content!After you view all the slides, you'll end up back here where you can click on the button to take the open book quiz. There are 20 questions pulled randomly from a pool of almost 100. Some questions are worth more points. You can view the slides anytime and take the quiz as many times as you need to.
Any video material will NOT be on the quiz. Click the right slide arrow to begin. The last slide has the link to the quiz.
How, why, when, and for what we vaccinate today starts with the story of smallpox, polio, and diphtheria. Our whole understanding of how vaccine immunity works began with the discovery of the relationship between smallpox and cowpox. This one thing alone resulted in an inestimable number of lives saved in the last two hundred years.This course is designed to give accurate information for parents. We recognize (and support) the right to refuse vaccines contrary to medical advice. But we also know that many times, parents seem to opt out because they don't know the facts about what vaccines do, what they actually contain, and why we think they are so important.It is best viewed on a desktop computer, and I strongly encourage you to take notes. I do not suggest that children view the content!After you view all the slides, you'll end up back here where you can click on the button to take the open book quiz. There are 20 questions pulled randomly from a pool of almost 100. Some questions are worth more points. You can view the slides anytime and take the quiz as many times as you need to.
Any video material will NOT be on the quiz. Click the right slide arrow to begin. The last slide has the link to the quiz.

Copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.
Copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.
All slides copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.

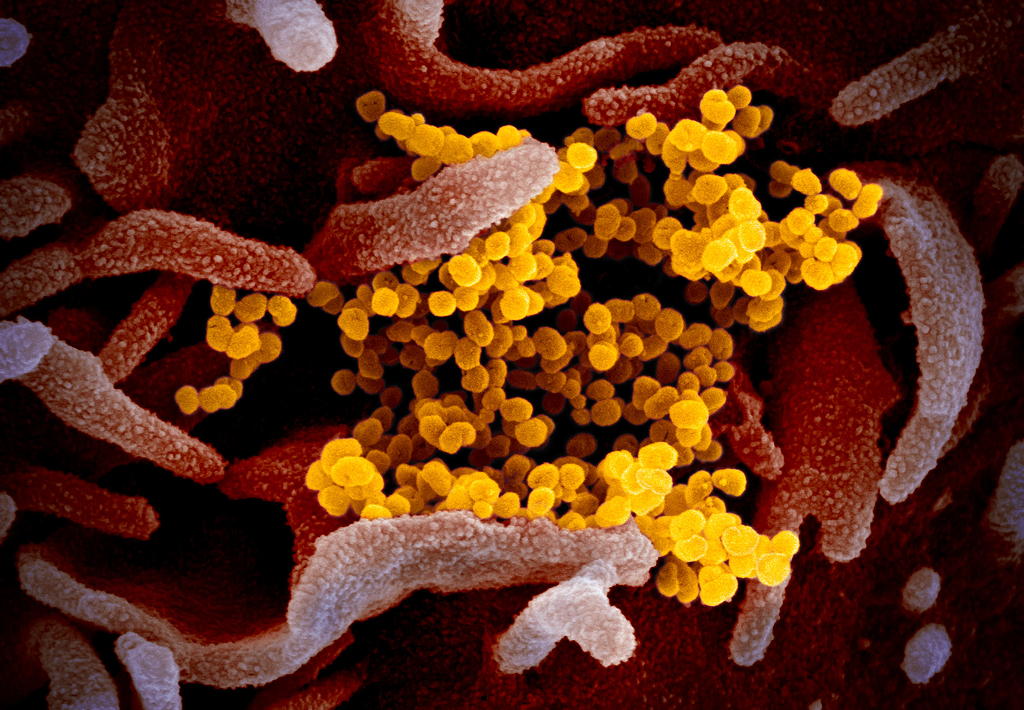
Background: This scanning electron microscope image shows SARS-CoV-2 (yellow)—also known as 2019-nCoV, the virus that causes COVID-19—isolated from a patient in the U.S., emerging from the surface of cells (pink) cultured in the lab. Image captured and colorized at NIAID's Rocky Mountain Laboratories (RML) in Hamilton, Montana. Credit: NIAID
Background: This scanning electron microscope image shows SARS-CoV-2 (yellow)—also known as 2019-nCoV, the virus that causes COVID-19—isolated from a patient in the U.S., emerging from the surface of cells (pink) cultured in the lab. Image captured and colorized at NIAID's Rocky Mountain Laboratories (RML) in Hamilton, Montana. Credit: NIAID
Background: This scanning electron microscope image shows SARS-CoV-2 (yellow)—also known as 2019-nCoV, the virus that causes COVID-19—isolated from a patient in the U.S., emerging from the surface of cells (pink) cultured in the lab. Image captured and colorized at NIAID's Rocky Mountain Laboratories (RML) in Hamilton, Montana. Credit: NIAID
Edward Jenner’s vaccine for smallpox was actually a whole, live cowpox virus. It is amazing that the vaccine he created worked, because most viruses do not have such similar structural relationship. Only because of that similarity was it effective. His stumbling discovery was the beginning of all future investigations and understanding into contagious infectious disease.
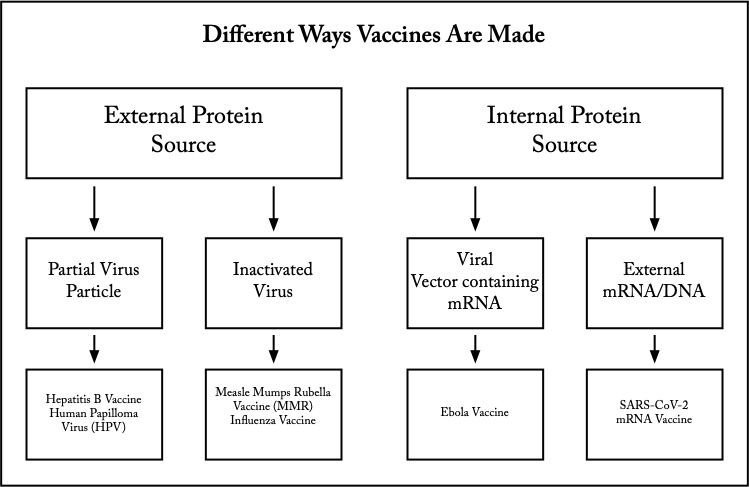
His vaccine was from an external protein source and is known as a whole pathogen vaccine. Unlike smallpox, cowpox didn’t cause serious illness. Most whole pathogen vaccines use inactivated (killed) sources of virus or bacteria. Viruses are inactivated with formaldehyde or heat. They have far fewer proteins than whole cell vaccines like the initial Pertussis (whooping cough) vaccine. Whole cell bacterial vaccines caused far more secondary reactions as a result. Using newer vaccine manufacturing methods have significantly reduced adverse reactions such as fever.
Inactivation, however, reduces the ability of the vaccine to induce host immunity without adding adjuvants like aluminum. Adjuvants increase vaccine effectiveness and reduce the required volume of injected vaccine. Measles-Mumps-Rubella and Influenza vaccines are inactivated virus vaccines.
With the polio vaccine, we discovered the importance of being sure of complete inactivation of whole pathogen vaccines. The failed virus inactivation at one manufacturer became known as the "Cutter incident." Live virus was discovered “hiding” in the vaccine because of the type of filters they were using. Children vaccinated with certain lots of the Cutter vaccine actually contracted polio and some become paralyzed. While no one wanted this, especially Cutter Labs, it highlighted the importance of being sure that whole pathogen vaccines were fully killed by the inactivation process.
Edward Jenner’s vaccine for smallpox was actually a whole, live cowpox virus. It is amazing that the vaccine he created worked, because most viruses do not have such similar structural relationship. Only because of that similarity was it effective. His stumbling discovery was the beginning of all future investigations and understanding into contagious infectious disease.
His vaccine was from an external protein source and is known as a whole pathogen vaccine. Unlike smallpox, cowpox didn’t cause serious illness. Most whole pathogen vaccines use inactivated (killed) sources of virus or bacteria. Viruses are inactivated with formaldehyde or heat. They have far fewer proteins than whole cell vaccines like the initial Pertussis (whooping cough) vaccine. Whole cell bacterial vaccines caused far more secondary reactions as a result. Using newer vaccine manufacturing methods have significantly reduced adverse reactions such as fever.
Inactivation, however, reduces the ability of the vaccine to induce host immunity without adding adjuvants like aluminum. Adjuvants increase vaccine effectiveness and reduce the required volume of injected vaccine. Measles-Mumps-Rubella and Influenza vaccines are inactivated virus vaccines.
With the polio vaccine, we discovered the importance of being sure of complete inactivation of whole pathogen vaccines. The failed virus inactivation at one manufacturer became known as the "Cutter incident." Live virus was discovered “hiding” in the vaccine because of the type of filters they were using. Children vaccinated with certain lots of the Cutter vaccine actually contracted polio and some become paralyzed. While no one wanted this, especially Cutter Labs, it highlighted the importance of being sure that whole pathogen vaccines were fully killed by the inactivation process.
Edward Jenner’s vaccine for smallpox was actually a whole, live cowpox virus. It is amazing that the vaccine he created worked, because most viruses do not have such similar structural relationship. Only because of that similarity was it effective. His stumbling discovery was the beginning of all future investigations and understanding into contagious infectious disease.
His vaccine was from an external protein source and is known as a whole pathogen vaccine. Unlike smallpox, cowpox didn’t cause serious illness. Most whole pathogen vaccines use inactivated (killed) sources of virus or bacteria. Viruses are inactivated with formaldehyde or heat. They have far fewer proteins than whole cell vaccines like the initial Pertussis (whooping cough) vaccine. Whole cell bacterial vaccines caused far more secondary reactions as a result. Using newer vaccine manufacturing methods have significantly reduced adverse reactions such as fever.
Inactivation, however, reduces the ability of the vaccine to induce host immunity without adding adjuvants like aluminum. Adjuvants increase vaccine effectiveness and reduce the required volume of injected vaccine. Measles-Mumps-Rubella and Influenza vaccines are inactivated virus vaccines.
With the polio vaccine, we discovered the importance of being sure of complete inactivation of whole pathogen vaccines. The failed virus inactivation at one manufacturer became known as the "Cutter incident." Live virus was discovered “hiding” in the vaccine because of the type of filters they were using. Children vaccinated with certain lots of the Cutter vaccine actually contracted polio and some become paralyzed. While no one wanted this, especially Cutter Labs, it highlighted the importance of being sure that whole pathogen vaccines were fully killed by the inactivation process.

Copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.
Copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.
All slides copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.

Background: This scanning electron microscope image shows SARS-CoV-2 (yellow)—also known as 2019-nCoV, the virus that causes COVID-19—isolated from a patient in the U.S., emerging from the surface of cells (pink) cultured in the lab. Image captured and colorized at NIAID's Rocky Mountain Laboratories (RML) in Hamilton, Montana. Credit: NIAID
Background: This scanning electron microscope image shows SARS-CoV-2 (yellow)—also known as 2019-nCoV, the virus that causes COVID-19—isolated from a patient in the U.S., emerging from the surface of cells (pink) cultured in the lab. Image captured and colorized at NIAID's Rocky Mountain Laboratories (RML) in Hamilton, Montana. Credit: NIAID
Background: This scanning electron microscope image shows SARS-CoV-2 (yellow)—also known as 2019-nCoV, the virus that causes COVID-19—isolated from a patient in the U.S., emerging from the surface of cells (pink) cultured in the lab. Image captured and colorized at NIAID's Rocky Mountain Laboratories (RML) in Hamilton, Montana. Credit: NIAID
Inactivated Virus or Bacteria.
The similarity between smallpox and cowpox will perhaps never be repeated. As a result, vaccine solutions for infections like polio and pertussis (whooping cough) moved to using inactivated (also called attenuated) virus and bacteria that could not replicate. The virus or bacteria are essentially killed.
This however was fraught with its own side effects. The DTwP (diphtheria toxin, tetanus toxin, and whole pertussis) vaccine used the entire pertussis bacteria. It was not uncommon to see children with fevers to 103-104°F which came from the myriad of proteins on and in the pertussis cell. The DTaP (with acellular pertussis) vaccine used only some of the proteins, yet the vaccine was equally effective at providing protection.
Partial Virus or Bacteria.
What this meant is that only parts (subunits) of the target virus or bacteria need be used to produce immunity. Later vaccines like the Hib (hemophilus influenza type b bacteria) used this technique and have been famously effective and preventing severe disease with extremely rare side effects. For viral vaccines, it meant that the Cutter incident would never occur since only intact virus within a capsid is infectious.
The only problem with these kinds of vaccines is the length of time to come up with a candidate vaccine which then has to go through Phase I, II, III, and sometimes IV trials. Many trial years leave some victims with serious and permanent residual effects while this testing proceeds. The amazing results with smallpox have not been repeated.
Inactivated Virus or Bacteria.
The similarity between smallpox and cowpox will perhaps never be repeated. As a result, vaccine solutions for infections like polio and pertussis (whooping cough) moved to using inactivated (also called attenuated) virus and bacteria that could not replicate. The virus or bacteria are essentially killed.
This however was fraught with its own side effects. The DTwP (diphtheria toxin, tetanus toxin, and whole pertussis) vaccine used the entire pertussis bacteria. It was not uncommon to see children with fevers to 103-104°F which came from the myriad of proteins on and in the pertussis cell. The DTaP (with acellular pertussis) vaccine used only some of the proteins, yet the vaccine was equally effective at providing protection.
Partial Virus or Bacteria.
What this meant is that only parts (subunits) of the target virus or bacteria need be used to produce immunity. Later vaccines like the Hib (hemophilus influenza type b bacteria) used this technique and have been famously effective and preventing severe disease with extremely rare side effects. For viral vaccines, it meant that the Cutter incident would never occur since only intact virus within a capsid is infectious.
The only problem with these kinds of vaccines is the length of time to come up with a candidate vaccine which then has to go through Phase I, II, III, and sometimes IV trials. Many trial years leave some victims with serious and permanent residual effects while this testing proceeds. The amazing results with smallpox have not been repeated.
Inactivated Virus or Bacteria.
The similarity between smallpox and cowpox will perhaps never be repeated. As a result, vaccine solutions for infections like polio and pertussis (whooping cough) moved to using inactivated (also called attenuated) virus and bacteria that could not replicate. The virus or bacteria are essentially killed.
This however was fraught with its own side effects. The DTwP (diphtheria toxin, tetanus toxin, and whole pertussis) vaccine used the entire pertussis bacteria. It was not uncommon to see children with fevers to 103-104°F which came from the myriad of proteins on and in the pertussis cell. The DTaP (with acellular pertussis) vaccine used only some of the proteins, yet the vaccine was equally effective at providing protection.
Partial Virus or Bacteria.
What this meant is that only parts (subunits) of the target virus or bacteria need be used to produce immunity. Later vaccines like the Hib (hemophilus influenza type b bacteria) used this technique and have been famously effective and preventing severe disease with extremely rare side effects. For viral vaccines, it meant that the Cutter incident would never occur since only intact virus within a capsid is infectious.
The only problem with these kinds of vaccines is the length of time to come up with a candidate vaccine which then has to go through Phase I, II, III, and sometimes IV trials. Many trial years leave some victims with serious and permanent residual effects while this testing proceeds. The amazing results with smallpox have not been repeated.

Copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.
Copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.
All slides copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.

Background: This scanning electron microscope image shows SARS-CoV-2 (yellow)—also known as 2019-nCoV, the virus that causes COVID-19—isolated from a patient in the U.S., emerging from the surface of cells (pink) cultured in the lab. Image captured and colorized at NIAID's Rocky Mountain Laboratories (RML) in Hamilton, Montana. Credit: NIAID
External vs Internal Protein Sources for Vaccines.
The partial or subunit protein sources clearly had the advantage over inactivated methods of vaccine development. Still, the time frame for vaccine development was essentially unchanged. Virus for vaccines still required a living host cell to make the multitude of copies required for the mass vaccination of the US population, let alone the world.
The next idea was to use the vaccinated person’s own cellular machinery to produce antigenic protein internally. Injection of the subunit of the target virus or bacteria which produced the immune response was no longer required. Rather, the specific mRNA (or DNA) from the virus or bacteria which coded for the important subunit would be injected.
The advances in genomic analysis in the recent decade have significantly reduced the time to go from subunit protein, to the mRNA that codes for that protein. Cell strains and living tissues, like fertilized chicken eggs, are no longer needed. While the mRNA is very short, it is also extremely temperature sensitive, requiring temperatures of -70° to maintain biologic activity.
Viral Vector Injection of mRNA/DNA.
The cyclic scourge of Ebola in Africa which kills a majority of victims lead to the development of a viral vector vaccine. The mRNA/DNA was inserted into the vector virus DNA. That vector virus would then be injected into the patient where it would infect the host cells. The vector virus itself was one chosen for its harmlessness, so it didn’t cause illness. However, the mRNA/DNA would be carried within the host cell where the ribosomes and tRNA (transfer RNA) would proceed to produce the target viral subunit protein.
Because of this complicated switcheroo, the person getting the vaccine would develop an immunity to the target virus.
External vs Internal Protein Sources for Vaccines.
The partial or subunit protein sources clearly had the advantage over inactivated methods of vaccine development. Still, the time frame for vaccine development was essentially unchanged. Virus for vaccines still required a living host cell to make the multitude of copies required for the mass vaccination of the US population, let alone the world.
The next idea was to use the vaccinated person’s own cellular machinery to produce antigenic protein internally. Injection of the subunit of the target virus or bacteria which produced the immune response was no longer required. Rather, the specific mRNA (or DNA) from the virus or bacteria which coded for the important subunit would be injected.
The advances in genomic analysis in the recent decade have significantly reduced the time to go from subunit protein, to the mRNA that codes for that protein. Cell strains and living tissues, like fertilized chicken eggs, are no longer needed. While the mRNA is very short, it is also extremely temperature sensitive, requiring temperatures of -70° to maintain biologic activity.
Viral Vector Injection of mRNA/DNA.
The cyclic scourge of Ebola in Africa which kills a majority of victims lead to the development of a viral vector vaccine. The mRNA/DNA was inserted into the vector virus DNA. That vector virus would then be injected into the patient where it would infect the host cells. The vector virus itself was one chosen for its harmlessness, so it didn’t cause illness. However, the mRNA/DNA would be carried within the host cell where the ribosomes and tRNA (transfer RNA) would proceed to produce the target viral subunit protein.
Because of this complicated switcheroo, the person getting the vaccine would develop an immunity to the target virus.
External vs Internal Protein Sources for Vaccines.
The partial or subunit protein sources clearly had the advantage over inactivated methods of vaccine development. Still, the time frame for vaccine development was essentially unchanged. Virus for vaccines still required a living host cell to make the multitude of copies required for the mass vaccination of the US population, let alone the world.
The next idea was to use the vaccinated person’s own cellular machinery to produce antigenic protein internally. Injection of the subunit of the target virus or bacteria which produced the immune response was no longer required. Rather, the specific mRNA (or DNA) from the virus or bacteria which coded for the important subunit would be injected.
The advances in genomic analysis in the recent decade have significantly reduced the time to go from subunit protein, to the mRNA that codes for that protein. Cell strains and living tissues, like fertilized chicken eggs, are no longer needed. While the mRNA is very short, it is also extremely temperature sensitive, requiring temperatures of -70° to maintain biologic activity.
Viral Vector Injection of mRNA/DNA.
The cyclic scourge of Ebola in Africa which kills a majority of victims lead to the development of a viral vector vaccine. The mRNA/DNA was inserted into the vector virus DNA. That vector virus would then be injected into the patient where it would infect the host cells. The vector virus itself was one chosen for its harmlessness, so it didn’t cause illness. However, the mRNA/DNA would be carried within the host cell where the ribosomes and tRNA (transfer RNA) would proceed to produce the target viral subunit protein.
Because of this complicated switcheroo, the person getting the vaccine would develop an immunity to the target virus.

Copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.
Copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.
All slides copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.

Background: This scanning electron microscope image shows SARS-CoV-2 (yellow)—also known as 2019-nCoV, the virus that causes COVID-19—isolated from a patient in the U.S., emerging from the surface of cells (pink) cultured in the lab. Image captured and colorized at NIAID's Rocky Mountain Laboratories (RML) in Hamilton, Montana. Credit: NIAID
What follows is a general description of mRNA vaccine manufacture. The first step is to identify the section of DNA that codes for the spike protein on the viral capsid. The genome (DNA sequence) for SARS-CoV-2 is published and openly available to everyone. Once the specific spike protein code is identified, then the mRNA sequence from which it made is created.
The mRNA code is used to create its source DNA code. That DNA is made into a plasmid which is a circular DNA with only the master code for the mRNA which is used to create the spike protein. The plasmid then infects the E. coli bacteria. Plasmids in the world of bacteria are the equivalent of viruses in the animal world. E. coli is an abundant and easily obtainable bacteria. The plasmid DNA then hijacks the E. coli biochemical machinery. That DNA plasmid code begins pouring lots of mRNA which inturn codes for the target spike protein.
This mRNA is collecte, purified., and given a protective coating of lipids. Without the lipids, the mRNA would never get into the animal cells.Once inside the cell, the ribosomes, themselves a complex set of protein enzymes, read the vaccine mRNA just like normal mRNA that would come from the cell nucleus. Only the spike protein of the SARS-CoV-2 virus is produced and nothing else. All mRNA has a limited lifespan inside the cells and is quickly degraded.
The spike protein then is what the immune system detects as foreign, just as if the whole virus was present. Antibodies then are created which fend off any real virus attack in the future. A significant number of people seem to already have antibodies that block SARS-CoV-2 from infecting the cells, probably because of exposure to other of the numerous corornaviruses in the wild. Antibodies prevent infection of the cell, but the leftover viral DNA fragments can still produce a positive PCR test.
Mutations occur in all viruses, but this manufacturing process can be quickly adjusted much faster than those for traditional vaccines. The shortest vaccine production in history is the mumps vaccine which took four years to develop. That underscores the value and future of mRNA vaccines.
What follows is a general description of mRNA vaccine manufacture. The first step is to identify the section of DNA that codes for the spike protein on the viral capsid. The genome (DNA sequence) for SARS-CoV-2 is published and openly available to everyone. Once the specific spike protein code is identified, then the mRNA sequence from which it made is created.
The mRNA code is used to create its source DNA code. That DNA is made into a plasmid which is a circular DNA with only the master code for the mRNA which is used to create the spike protein. The plasmid then infects the E. coli bacteria. Plasmids in the world of bacteria are the equivalent of viruses in the animal world. E. coli is an abundant and easily obtainable bacteria. The plasmid DNA then hijacks the E. coli biochemical machinery. That DNA plasmid code begins pouring lots of mRNA which inturn codes for the target spike protein.
This mRNA is collecte, purified., and given a protective coating of lipids. Without the lipids, the mRNA would never get into the animal cells.Once inside the cell, the ribosomes, themselves a complex set of protein enzymes, read the vaccine mRNA just like normal mRNA that would come from the cell nucleus. Only the spike protein of the SARS-CoV-2 virus is produced and nothing else. All mRNA has a limited lifespan inside the cells and is quickly degraded.
The spike protein then is what the immune system detects as foreign, just as if the whole virus was present. Antibodies then are created which fend off any real virus attack in the future. A significant number of people seem to already have antibodies that block SARS-CoV-2 from infecting the cells, probably because of exposure to other of the numerous corornaviruses in the wild. Antibodies prevent infection of the cell, but the leftover viral DNA fragments can still produce a positive PCR test.
Mutations occur in all viruses, but this manufacturing process can be quickly adjusted much faster than those for traditional vaccines. The shortest vaccine production in history is the mumps vaccine which took four years to develop. That underscores the value and future of mRNA vaccines.
What follows is a general description of mRNA vaccine manufacture. The first step is to identify the section of DNA that codes for the spike protein on the viral capsid. The genome (DNA sequence) for SARS-CoV-2 is published and openly available to everyone. Once the specific spike protein code is identified, then the mRNA sequence from which it is made is created.
The mRNA code is used to create its source DNA code. That DNA is made into a plasmid which is a circular DNA with only the master code for the mRNA which is used to create the spike protein. The plasmid then infects the E. coli bacteria. Plasmids in the world of bacteria are the equivalent of viruses in the animal world. E. coli is an abundant and easily obtainable bacteria. The plasmid DNA then hijacks the E. coli biochemical machinery. That DNA plasmid code begins pouring lots of mRNA which inturn codes for the target spike protein.
This mRNA is collected, purified., and given a protective coating of lipids. Without the lipids, the mRNA would never get into the animal cells.Once inside the cell, the ribosomes, themselves a complex set of protein enzymes, read the vaccine mRNA just like normal mRNA that would come from the cell nucleus. Only the spike protein of the SARS-CoV-2 virus is produced and nothing else. All mRNA has a limited lifespan inside the cells and is quickly degraded.
The spike protein then is what the immune system detects as foreign, just as if the whole virus was present. Antibodies then are created which fend off any real virus attack in the future. A significant number of people seem to already have antibodies that block SARS-CoV-2 from infecting the cells, probably because of exposure to other of the numerous corornaviruses in the wild. Antibodies prevent infection of the cell, but the leftover viral DNA fragments can still produce a positive PCR test.
Mutations occur in all viruses, but this manufacturing process can be quickly adjusted much faster than those for traditional vaccines. The shortest vaccine production in history is the mumps vaccine which took four years to develop. That underscores the value and future of mRNA vaccines.

Copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.
Copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.
All slides copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.

Background: This scanning electron microscope image shows SARS-CoV-2 (yellow)—also known as 2019-nCoV, the virus that causes COVID-19—isolated from a patient in the U.S., emerging from the surface of cells (pink) cultured in the lab. Image captured and colorized at NIAID's Rocky Mountain Laboratories (RML) in Hamilton, Montana. Credit: NIAID
Direct Injection of External mRNA
We now can produce mRNA/DNA strands that code for the important subunit proteins that give our immune system the ability to fight those infections without ever having suffered the diseases themselves. The time from the identification of the best subunit proteins in the virus or bacteria to producing the necessary mRNA/DNA which codes for it is down to a handful of months or less.
Undoubtedly this is the frontier for vaccines and infectious diseases. We are running out of effective antibiotics as fast as we can find new ones. The last, best defense––our own immune system––must become the front line. This will be how we defeat such things as MRSA (multiply resistant staph aureus) and flesh-eating bacteria.
Clinical Trial and Testing
All vaccines require actual clinical trial and testing. Phase I testing involves vaccinating a very small number of healthy people. If all goes well, then Phase II vaccinates a larger group of healthy people. Phase III then vaccinates a very large group of both healthy individuals and those with various chronic diseases.
Initially, these phases are not intended for those who need the protection. The first phases are intended to test the effectiveness of the vaccine and document any side effects. In the later phases, those actually needing the vaccine protection are included in the studies. Phase IV testing may or may not be done. It involves a very, very large number of individuals, and it is often done to show the side effects with the lowest likelihood of occurrence. Pharmaceutical companies often start Phase IV testing at their own discretion after the FDA, following a successful Phase III, has licensed the vaccine.
Direct Injection of External mRNA
We now can produce mRNA/DNA strands that code for the important subunit proteins that give our immune system the ability to fight those infections without ever having suffered the diseases themselves. The time from the identification of the best subunit proteins in the virus or bacteria to producing the necessary mRNA/DNA which codes for it is down to a handful of months or less.
Undoubtedly this is the frontier for vaccines and infectious diseases. We are running out of effective antibiotics as fast as we can find new ones. The last, best defense––our own immune system––must become the front line. This will be how we defeat such things as MRSA (multiply resistant staph aureus) and flesh-eating bacteria.
Clinical Trial and Testing
All vaccines require actual clinical trial and testing. Phase I testing involves vaccinating a very small number of healthy people. If all goes well, then Phase II vaccinates a larger group of healthy people. Phase III then vaccinates a very large group of both healthy individuals and those with various chronic diseases.
Initially, these phases are not intended for those who need the protection. The first phases are intended to test the effectiveness of the vaccine and document any side effects. In the later phases, those actually needing the vaccine protection are included in the studies. Phase IV testing may or may not be done. It involves a very, very large number of individuals, and it is often done to show the side effects with the lowest likelihood of occurrence. Pharmaceutical companies often start Phase IV testing at their own discretion after the FDA, following a successful Phase III, has licensed the vaccine.
Direct Injection of External mRNA
We now can produce mRNA/DNA strands that code for the important subunit proteins that give our immune system the ability to fight those infections without ever having suffered the diseases themselves. The time from the identification of the best subunit proteins in the virus or bacteria to producing the necessary mRNA/DNA which codes for it is down to a handful of months or less.
Undoubtedly this is the frontier for vaccines and infectious diseases. We are running out of effective antibiotics as fast as we can find new ones. The last, best defense––our own immune system––must become the front line. This will be how we defeat such things as MRSA (multiply resistant staph aureus) and flesh-eating bacteria.
Clinical Trial and Testing
All vaccines require actual clinical trial and testing. Phase I testing involves vaccinating a very small number of healthy people. If all goes well, then Phase II vaccinates a larger group of healthy people. Phase III then vaccinates a very large group of both healthy individuals and those with various chronic diseases.
Initially, these phases are not intended for those who need the protection. The first phases are intended to test the effectiveness of the vaccine and document any side effects. In the later phases, those actually needing the vaccine protection are included in the studies. Phase IV testing may or may not be done. It involves a very, very large number of individuals, and it is often done to show the side effects with the lowest likelihood of occurrence. Pharmaceutical companies often start Phase IV testing at their own discretion after the FDA, following a successful Phase III, has licensed the vaccine.

Copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.
Copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.
All slides copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.
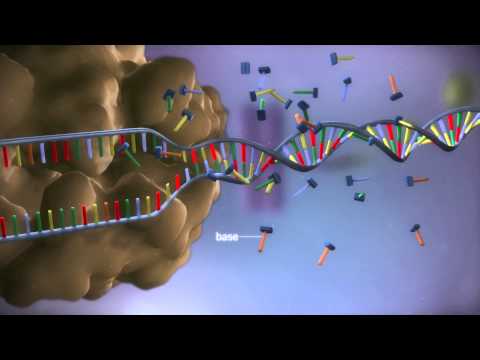
From DNA to Protein.
Used by permission under the Creative Commons Attribution 4.0 CC-BY License. Attribution: yourgenome, (2017). Copyright information available at: https://www.yourgenome.org/copyright.
Background: This scanning electron microscope image shows SARS-CoV-2 (yellow)—also known as 2019-nCoV, the virus that causes COVID-19—isolated from a patient in the U.S., emerging from the surface of cells (pink) cultured in the lab. Image captured and colorized at NIAID's Rocky Mountain Laboratories (RML) in Hamilton, Montana. Credit: NIAID
From DNA to Protein.
Used by permission under the Creative Commons Attribution 4.0 CC-BY License. Attribution: yourgenome, (2017). Copyright information available at: https://www.yourgenome.org/copyright.
Background: This scanning electron microscope image shows SARS-CoV-2 (yellow)—also known as 2019-nCoV, the virus that causes COVID-19—isolated from a patient in the U.S., emerging from the surface of cells (pink) cultured in the lab. Image captured and colorized at NIAID's Rocky Mountain Laboratories (RML) in Hamilton, Montana. Credit: NIAID
From DNA to Protein.
Used by permission under the Creative Commons Attribution 4.0 CC-BY License. Attribution: yourgenome, (2017). Copyright information available at: https://www.yourgenome.org/copyright.
Background: This scanning electron microscope image shows SARS-CoV-2 (yellow)—also known as 2019-nCoV, the virus that causes COVID-19—isolated from a patient in the U.S., emerging from the surface of cells (pink) cultured in the lab. Image captured and colorized at NIAID's Rocky Mountain Laboratories (RML) in Hamilton, Montana. Credit: NIAID
With the first vaccines for SARS-CoV-2 (Coronavirus 2019), you need to have a basic understanding of what mRNA is and what it does. You should watch the explainer video on this slide first.
The mRNA is called messenger RNA because it is a copy of a small gene section of DNA which contains all the master genes. Genes are the master maps, which are the instructions for the linking order of the amino acids that then make all our body’s proteins. Proteins then are the enzymes or structure building blocks which hold us together from the cellular level up to the skin, bones, muscles, ligaments, and organs.
When a virus enters the body, it is first floating free in the fluid between the cells. Viral particles are called capsids. They are extremely small compared to normal body cells and bacteria, and they only have the outer coating and a small strand of mRNA or DNA inside. These small strands of nucleic acids cannot replicate within the capsid like bacterial and normal body cells do. It is only when the capsid touches the outside membrane of the cell or bacteria that it can infect them. Once the virus is inside, it begins to multiply.
The proteins on the outside of the viral capsid attach to proteins on the surface of its target cell, and then the viral capsid fuses with the target cell membrane. The viral mRNA or DNA is then floating free within cell itself.
Viral mRNA then proceeds to the ribosome and makes the viral particle proteins. Either the viral capsids are released at the cell membrane, or the inside of the cell becomes filled with millions of copies of complete viral capsids. When the cell explodes and dies, it releases all the new virus particles.
With the first vaccines for SARS-CoV-2 (Coronavirus 2019), you need to have a basic understanding of what mRNA is and what it does. You should watch the explainer video on this slide first.
The mRNA is called messenger RNA because it is a copy of a small gene section of DNA which contains all the master genes. Genes are the master maps, which are the instructions for the linking order of the amino acids that then make all our body’s proteins. Proteins then are the enzymes or structure building blocks which hold us together from the cellular level up to the skin, bones, muscles, ligaments, and organs.
When a virus enters the body, it is first floating free in the fluid between the cells. Viral particles are called capsids. They are extremely small compared to normal body cells and bacteria, and they only have the outer coating and a small strand of mRNA or DNA inside. These small strands of nucleic acids cannot replicate within the capsid like bacterial and normal body cells do. It is only when the capsid touches the outside membrane of the cell or bacteria that it can infect them. Once the virus is inside, it begins to multiply.
The proteins on the outside of the viral capsid attach to proteins on the surface of its target cell, and then the viral capsid fuses with the target cell membrane. The viral mRNA or DNA is then floating free within cell itself.
Viral mRNA then proceeds to the ribosome and makes the viral particle proteins. Either the viral capsids are released at the cell membrane, or the inside of the cell becomes filled with millions of copies of complete viral capsids. When the cell explodes and dies, it releases all the new virus particles.
With the first vaccines for SARS-CoV-2 (Coronavirus 2019), you need to have a basic understanding of what mRNA is and what it does. You should watch the explainer video on this slide first.
The mRNA is called messenger RNA because it is a copy of a small gene section of DNA which contains all the master genes. Genes are the master maps, which are the instructions for the linking order of the amino acids that then make all our body’s proteins. Proteins then are the enzymes or structure building blocks which hold us together from the cellular level up to the skin, bones, muscles, ligaments, and organs.
When a virus enters the body, it is first floating free in the fluid between the cells. Viral particles are called capsids. They are extremely small compared to normal body cells and bacteria, and they only have the outer coating and a small strand of mRNA or DNA inside. These small strands of nucleic acids cannot replicate within the capsid like bacterial and normal body cells do. It is only when the capsid touches the outside membrane of the cell or bacteria that it can infect them. Once the virus is inside, it begins to multiply.
The proteins on the outside of the viral capsid attach to proteins on the surface of its target cell, and then the viral capsid fuses with the target cell membrane. The viral mRNA or DNA is then floating free within cell itself.
Viral mRNA then proceeds to the ribosome and makes the viral particle proteins. Either the viral capsids are released at the cell membrane, or the inside of the cell becomes filled with millions of copies of complete viral capsids. When the cell explodes and dies, it releases all the new virus particles.

Copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.
Copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.
All slides copyright © 2020 and beyond by Ron Smith, MD. All rights reserved.

From DNA to Protein.
Used by permission under the Creative Commons Attribution 4.0 CC-BY License. Attribution: yourgenome, (2017). Copyright information available at: https://www.yourgenome.org/copyright.
Background: This scanning electron microscope image shows SARS-CoV-2 (yellow)—also known as 2019-nCoV, the virus that causes COVID-19—isolated from a patient in the U.S., emerging from the surface of cells (pink) cultured in the lab. Image captured and colorized at NIAID's Rocky Mountain Laboratories (RML) in Hamilton, Montana. Credit: NIAID
From DNA to Protein.
Used by permission under the Creative Commons Attribution 4.0 CC-BY License. Attribution: yourgenome, (2017). Copyright information available at: https://www.yourgenome.org/copyright.
Background: This scanning electron microscope image shows SARS-CoV-2 (yellow)—also known as 2019-nCoV, the virus that causes COVID-19—isolated from a patient in the U.S., emerging from the surface of cells (pink) cultured in the lab. Image captured and colorized at NIAID's Rocky Mountain Laboratories (RML) in Hamilton, Montana. Credit: NIAID
From DNA to Protein.
Used by permission under the Creative Commons Attribution 4.0 CC-BY License. Attribution: yourgenome, (2017). Copyright information available at: https://www.yourgenome.org/copyright.
Background: This scanning electron microscope image shows SARS-CoV-2 (yellow)—also known as 2019-nCoV, the virus that causes COVID-19—isolated from a patient in the U.S., emerging from the surface of cells (pink) cultured in the lab. Image captured and colorized at NIAID's Rocky Mountain Laboratories (RML) in Hamilton, Montana. Credit: NIAID
Vaccines that use mRNA can act quickly just like a virus. The mRNA is quickly absorbed into host cells where it quickly starts using the ribosomes and tRNA (transfer RNA) to produce the target protein subunit. This target protein is processed through a series of immune cell interactions which concludes with the B-cell lymphocytes producing antibody against the target subunit protein.
B-cell lymphocytes begin life undifferentiated. That means they have never produced antibodies to any foreign infectious subunit. Once the B-cell becomes “aware” of this foreign subunit protein and produces antibodies to it, it can become less active and produces less antibody over time. This differentiated B-cell will always retain a memory of how to produce that antibody. It will also produce antibodies to only that one foreign protein. It cannot produce antibodies to other foreign infectious proteins.
This is important because sometimes immunity seems to wane over time. The key here though is how we are measuring that immunity. If we are measuring antibody levels against an infection, we cannot be exactly sure that we have lost the memory of how to make those antibodies.
This may be important, for example, in smallpox. Currently vaccine levels are present for about seven years before they wane. What we don’t know then is if smallpox were to break out again, would those like myself who was vaccinated in the 60s be able to mount an immune response and ramp the antibody production back up.
Will mRNA vaccines be able to improve this? Perhaps. One thing is for sure. If we have the genome for an infection, we have much greater leeway to develop a current vaccine to replace older, possibly ineffective, stockpiles for infections like smallpox that we don’t even see in the wild.
Vaccines that use mRNA can act quickly just like a virus. The mRNA is quickly absorbed into host cells where it quickly starts using the ribosomes and tRNA (transfer RNA) to produce the target protein subunit. This target protein is processed through a series of immune cell interactions which concludes with the B-cell lymphocytes producing antibody against the target subunit protein.
B-cell lymphocytes begin life undifferentiated. That means they have never produced antibodies to any foreign infectious subunit. Once the B-cell becomes “aware” of this foreign subunit protein and produces antibodies to it, it can become less active and produces less antibody over time. This differentiated B-cell will always retain a memory of how to produce that antibody. It will also produce antibodies to only that one foreign protein. It cannot produce antibodies to other foreign infectious proteins.
This is important because sometimes immunity seems to wane over time. The key here though is how we are measuring that immunity. If we are measuring antibody levels against an infection, we cannot be exactly sure that we have lost the memory of how to make those antibodies.
This may be important, for example, in smallpox. Currently vaccine levels are present for about seven years before they wane. What we don’t know then is if smallpox were to break out again, would those like myself who was vaccinated in the 60s be able to mount an immune response and ramp the antibody production back up.
Will mRNA vaccines be able to improve this? Perhaps. One thing is for sure. If we have the genome for an infection, we have much greater leeway to develop a current vaccine to replace older, possibly ineffective, stockpiles for infections like smallpox that we don’t even see in the wild.
Vaccines that use mRNA can act quickly just like a virus. The mRNA is quickly absorbed into host cells where it quickly starts using the ribosomes and tRNA (transfer RNA) to produce the target protein subunit. This target protein is processed through a series of immune cell interactions which concludes with the B-cell lymphocytes producing antibody against the target subunit protein.
B-cell lymphocytes begin life undifferentiated. That means they have never produced antibodies to any foreign infectious subunit. Once the B-cell becomes “aware” of this foreign subunit protein and produces antibodies to it, it can become less active and produces less antibody over time. This differentiated B-cell will always retain a memory of how to produce that antibody. It will also produce antibodies to only that one foreign protein. It cannot produce antibodies to other foreign infectious proteins.
This is important because sometimes immunity seems to wane over time. The key here though is how we are measuring that immunity. If we are measuring antibody levels against an infection, we cannot be exactly sure that we have lost the memory of how to make those antibodies.
This may be important, for example, in smallpox. Currently vaccine levels are present for about seven years before they wane. What we don’t know then is if smallpox were to break out again, would those like myself who was vaccinated in the 60s be able to mount an immune response and ramp the antibody production back up.
Will mRNA vaccines be able to improve this? Perhaps. One thing is for sure. If we have the genome for an infection, we have much greater leeway to develop a current vaccine to replace older, possibly ineffective, stockpiles for infections like smallpox that we don’t even see in the wild.
